HEALTH NEWS
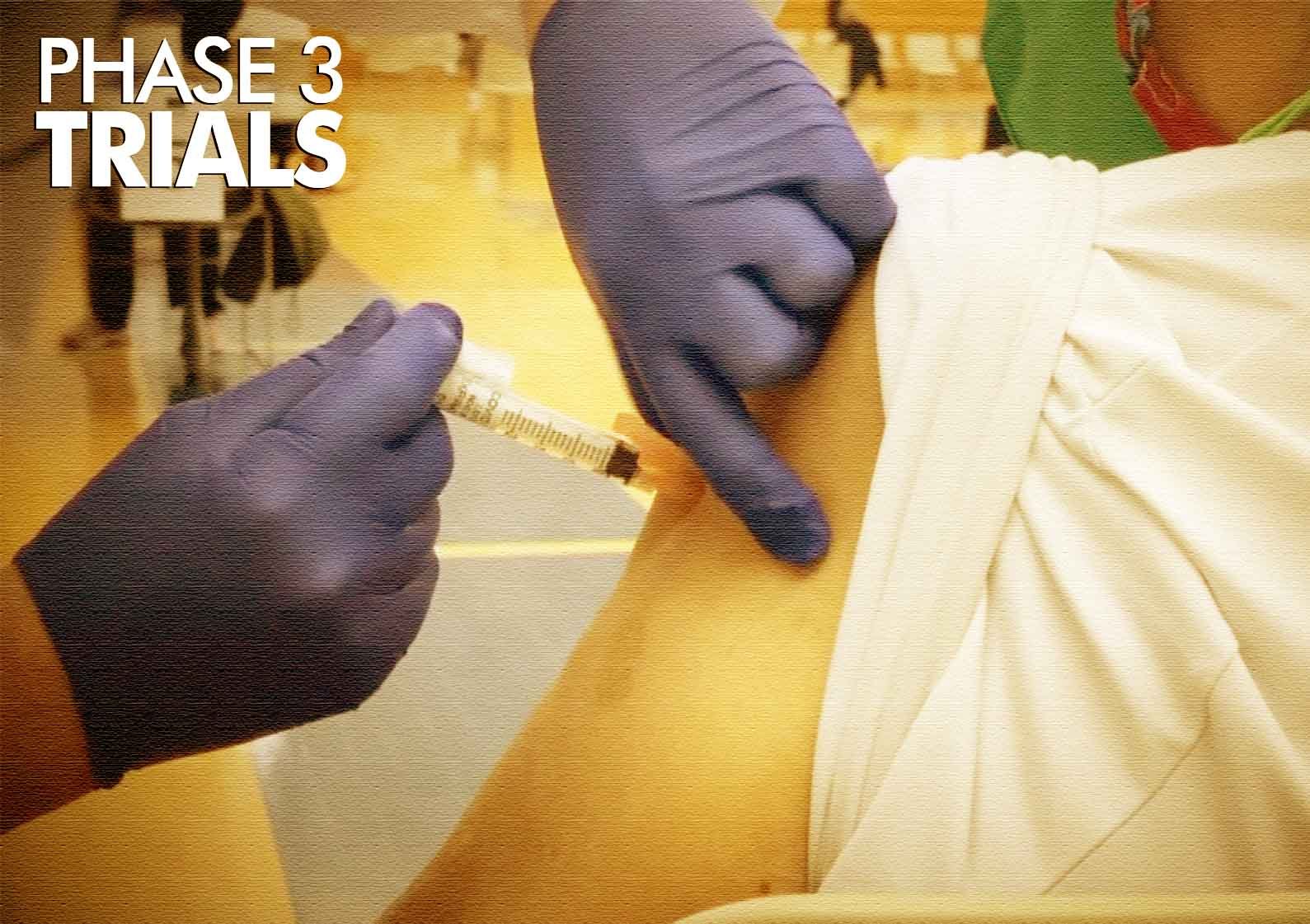
Promising Advances: Cancer Vaccine Enters Phase 3 Clinical Trials
-
Rahul Priydarss
I
n a groundbreaking development in the field of oncology, a potential cancer vaccine has reached the crucial Phase 3 clinical trial stage
Table of Contents
Introduction:
In a groundbreaking development in the field of oncology, a potential cancer vaccine has reached the crucial Phase 3 clinical trial stage, marking a significant stride towards a novel and effective approach to cancer treatment. This vaccine, designed to target specific cancer cells and bolster the body’s immune response, holds promise for transforming the landscape of cancer therapies.
Background:
Cancer continues to be a formidable adversary, affecting millions of lives worldwide. Conventional treatments, such as chemotherapy and radiation, have been the standard of care for decades. However, these treatments often come with severe side effects and may not provide a complete cure. The emergence of immunotherapy, harnessing the body’s immune system to combat cancer, has offered new hope.
The Vaccine:
▪ The cancer vaccine under scrutiny is designed to train the immune system to recognize and attack cancer cells. Unlike traditional treatments, which directly target cancer cells or inhibit their growth, immunotherapy aims to empower the body’s natural defences. The vaccine, developed after years of meticulous research, targets specific antigens present in cancer cells, effectively marking them for destruction by the immune system.
▪ The most recent findings, presented at an academic conference, revealed that nearly 95% of individuals who exclusively received the vaccine were still alive three years into treatment, with 64% maintaining a disease-free status.
In cases of advanced melanoma, the vaccine-only group demonstrated a 60% disease-free survival rate after three years for individuals with stage III disease, compared to approximately 39% in the placebo group. For those with stage IV disease, the vaccine-only group exhibited a disease-free survival rate of about 68%, while the placebo group reported zero.
“Notable side effects included redness or pain at the injection site, along with post-injection fever and fatigue—common reactions akin to other vaccines that stimulate an immune response.”
▪ Dr. Vernon Sondak, a cutaneous oncologist at Moffitt Cancer Center not involved in the trial but experienced with tumour lysate vaccines, shared with ABC News that these results are promising. However, he highlighted the non-conclusive nature of Phase 2 clinical trials.
▪ Dr. Sondak emphasized that the true impact of this cancer vaccine as a game-changer in the field hinges on the outcomes of a more extensive Phase 3 clinical trial. He cautioned against premature optimism, noting the history of promising Phase 2 data not necessarily translating into success during Phase 3 trials.
Progress to Phase 3:
▪ The transition to Phase 3 clinical trials is a pivotal moment in the vaccine’s development. Phase 3 trials involve a large and diverse group of participants, typically numbering in the thousands, to rigorously evaluate the vaccine’s safety and efficacy. This final phase is crucial in determining whether the vaccine can deliver the anticipated benefits on a broader scale.
▪ The trials will assess various factors, including the vaccine’s ability to induce a robust immune response, its impact on tumour reduction, and its overall safety profile. Researchers will closely monitor participants for any adverse effects and evaluate the long-term effectiveness of the vaccine.
Implications for Cancer Treatment:
▪ If successful, this cancer vaccine could revolutionize cancer treatment protocols. Unlike traditional therapies, which often come with debilitating side effects, immunotherapy has shown the potential for fewer adverse reactions while providing durable responses. The specificity of the vaccine, targeting cancer cells without harming healthy tissue, is a key advantage that may enhance its safety profile.
▪ Moreover, the vaccine’s ability to stimulate the immune system could have broader applications, potentially preventing cancer recurrence and offering a preventive strategy for high-risk individuals.
▪ The prospect of a cancer vaccine reaching Phase 3 trials is generating excitement among healthcare professionals, researchers, and, most importantly, patients and their families who are eagerly awaiting innovative and more effective treatment options.
Challenges and Future Directions:
While the progression to Phase 3 trials is a significant milestone, challenges remain. The vaccine’s success is not guaranteed, and further research is needed to understand its efficacy across different cancer types and patient populations. Additionally, the logistical aspects of manufacturing, distribution, and affordability will play crucial roles in determining the vaccine’s accessibility to a broader population.

Important info about Cancer Vaccine trials and What Doctors Say:
Survival Rates:
▪ The data presented at an academic conference indicates that nearly 95% of individuals who exclusively received the vaccine were still alive three years into treatment.
▪ Disease-free survival rates are noteworthy, with 64% of individuals remaining disease-free after the same duration.
Advanced Melanoma Breakdown:
In the most advanced forms of melanoma:
▪ For individuals with stage III disease, the vaccine-only group exhibited a 60% disease-free survival rate after three years, compared to about 39% in the placebo group.
▪ Those with stage IV disease in the vaccine-only group showed a disease-free survival rate of approximately 68%, while the placebo group reported zero.
Common Side Effects:
▪ The most common side effects of the vaccine included redness or pain at the injection site, along with post-injection fever and fatigue.
▪ These reactions are consistent with those observed in vaccines that stimulate an immune response.
Expert Commentary – Dr. Vernon Sondak:
▪ Dr. Vernon Sondak, a cutaneous oncologist at Moffitt Cancer Center, although not directly involved in the clinical trial, views the results as promising.
▪ He cautions that while Phase 2 clinical trials show promise, they are not conclusive. Dr. Sondak emphasizes the need for a larger Phase 3 clinical trial to validate the potential game-changing impact of the cancer vaccine.
Cautionary Note on Phase 2 vs. Phase 3 Data:
▪ Dr. Sondak points out a historical pattern where promising Phase 2 data does not always translate into success during Phase 3 trials.
▪ This cautionary note underscores the importance of conducting larger and more comprehensive trials to substantiate the initial findings.
Frequently Asked Questions (FAQs):
Q1: Why is the Phase 3 clinical trial significant for the cancer vaccine’s development?
A1: Phase 3 trials are crucial as they rigorously evaluate the safety and efficacy of the cancer vaccine on a larger scale, providing critical data for regulatory approval and potential transformative impacts on cancer treatment.
Q2: How does this cancer vaccine differ from traditional treatments like chemotherapy?
A2: Unlike traditional treatments that directly target cancer cells, this vaccine harnesses the immune system, offering a targeted approach with the potential benefits of fewer side effects and a more specific response against cancer cells.
Q3: What potential advantages do immunotherapy, represented by this vaccine, hold for cancer patients?
A3: Immunotherapy, exemplified by this vaccine, has shown promise in providing durable responses with fewer adverse reactions compared to conventional treatments, making it a potential game-changer in the landscape of cancer care.
Q4: What challenges does the cancer vaccine face, and what factors affect its future accessibility?
A4: Despite reaching Phase 3, challenges include uncertainties about success and the need for further research on its efficacy. Logistical aspects such as manufacturing, distribution, and affordability will determine the vaccine’s accessibility if it receives regulatory approval.
-Remember, Always consult with healthcare professionals or Doctors for personalised advice related to medical conditions.
Conclusion:
The advancement of the cancer vaccine to Phase 3 clinical trials represents a beacon of hope in the quest for effective and targeted cancer treatments. As the trials unfold, the healthcare community eagerly anticipates the results that could potentially redefine the future of cancer care. The collective efforts of researchers, clinicians, and pharmaceutical companies in pushing the boundaries of medical science underscore the relentless pursuit of innovative solutions to combat one of humanity’s most challenging adversaries.
Previous Post